Bilio-Pancreatic Strictures
Bilio-Pancreatic Strictures
Cellvizio® is the real-time in vivo cellular imaging platform that enables you to characterize areas of interest with confidence in Bilio-Pancreatic Strictures in cases where conventional disease screening returns ambiguous results.
Live unlimited tissue assessment with Cellvizio® enables superior characterization of indeterminate biliary and pancreatic strictures
Bilio-pancreatic strictures remain very difficult to diagnose due to the difficulty of getting adequate tissue samples. With Cellvizio®, you can see the stricture at the microscopic level, in real-time during the ERCP procedure, which enables more accurate and instantaneous classification.1,2
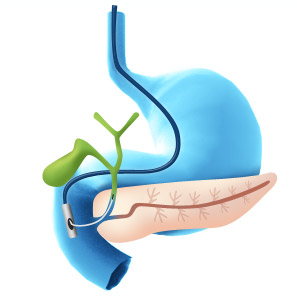
This translates into the ability to detect more malignant strictures during initial ERCP procedure, thus reducing repeat procedures and offering patients an earlier and more informed treatment option. It also makes it possible for the physician to rule out disease and send a patient home with high confidence. 1,3
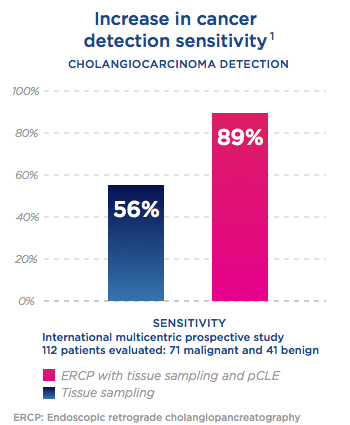
Higher detection of Cancer in ERCP
with Cellvizio®
Higher detection of
Cancer in ERCP
with Cellvizio®
The newer endoscopic imaging modality of CLE has kindled an interest in the field of advanced imaging offering a real-time histopathologic evaluation of the pancreaticobiliary system. It has strengthened and extended the arm of the gastroenterologist from a therapeutic endoscopist to an endopathologist. The novel use of this technique is particularly of significance in differentiating indeterminate biliary strictures as treatment depends on an accurate and prompt diagnosis.
Pr. Shajan Peter
University Hospital Basel, Switzerland
Confocal Laser Endomicroscopy by Cellvizio® is a through-the-scope procedure that fits any standard practice and equipment
Confocal Laser Endomicroscopy
by Cellvizio®
is a through-the-scope procedure
that fits any standard practice and equipment
Benign Stricture
Visualized with Cellvizio® CholangioFlex miniprobe4
Cholangiocarcinoma
Visualized with Cellvizio® CholangioFlex miniprobe4
References & disclaimers
1. Meining A. et al. Direct Visualization of Indeterminate Pancreaticobiliary Strictures using Probe-based Confocal Laser Endomicroscopy – A Multicenter Experience.
Gastrointestinal Endoscopy, 2011.
2. Meining A. et al. Classification of Probe-based Confocal Laser Endomicroscopy findings in Pancreaticobiliary Strictures. Endoscopy, 2012.
3. Caillol F, Bories E, Autret A, Poizat F, Pesenti C, Ewald J, Turrini O, Delpero JR, Monges G, Giovannini M. Evaluation of pCLE in the bile duct: final results of EMID study : pCLE: impact in the management of bile duct strictures. Surg Endosc. 2014 Dec 10.
4. Slivka et al. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy (pCLE) for the characterization of indeterminate biliary strictures: results of a prospective multicenter. GIE, 2015
5. Caillol et al. Refined Probe-Based Confocal Laser Endomicroscopy Classification for Biliary Strictures: The Paris Classification. Dig Dis Sciences 2013
Cellvizio® I.V.E. with Confocal MiniprobesTM are regulated Medical Device, CE marked (CE 0459) (Class IIa – NB : G-MED) and FDA cleared. Cellvizio® is a registered trademark and Confocal MiniprobeTM is a trademark of Mauna Kea Technologies. The Cellvizio® I.V.E. is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. Once connected to the Cellvizio® I.V.E.: the CholangioFlexTM N Confocal MiniprobesTM are intended to allow imaging of the upper gastrointestinal tract including biliary and pancreatic ducts accessed by an endoscope or endoscopic accessories. Please consult labels and instructions for use. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. The use of this medical device is exclusively reserved for health professionals. Product availability cannot be guaranteed in all countries. For further information, please contact your local sales representative.
News
YOU MIGHT ALSO LIKE TO READ

Mauna Kea Technologies announces results of its Combined General Meeting of June 5, 2025
Mauna Kea Technologies Announces Major AI Breakthrough with Cellvizio in Pancreatic Cystic Lesion Risk Stratification

Mauna Kea Technologies Provides Update on Safeguard Proceedings, Strategic Discussions and Operational Progress
Find out more by downloading the
Cellvizio Brochure
