Colorectal Lesions
Cellvizio® is the real-time in vivo cellular imaging platform that enables you to detect and monitor the progression of colorectal lesions in vivo over time using proprietary advanced imaging technology.
With the help of endomicroscopy, physicians can also better detect residual neoplasia following EMR as compared with WLE, and adapt the re-treatment modalities if needed.1,2,3,4,6
Cellvizio enables you to monitor tissue at the cellular level during standard endoscopies, which helps differentiate between non-neoplastic and neoplastic tissue. This technique allows for a real-time characterization of polyps during screening colonoscopies and characterization of lesions at risk for cancer.
With the help of endomicroscopy, physicians can also better detect residual neoplasia following EMR as compared with WLE, and adapt the re-treatment modalities if needed. 1,2,3,4,6
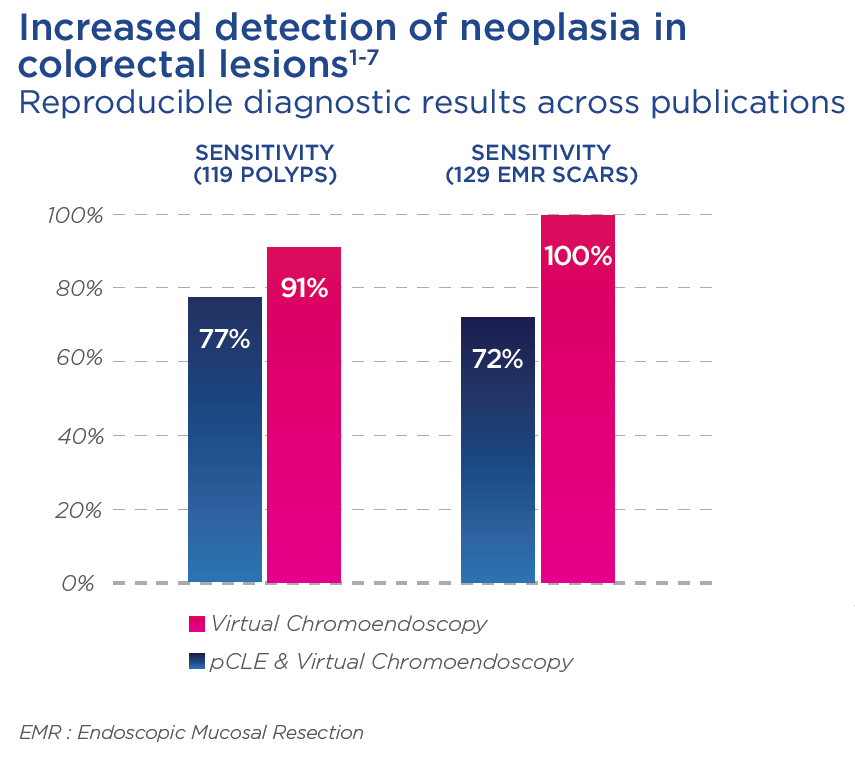
The addition of pCLE provides higher sensitivity and excellent NPV, thus allowing a highly accurate decision to not treat again in cases where lesions appear to be non-neoplastic.
Muhammad W. Shahid, MD
Mayo Clinic, Jacksonville, FL, USA2
Cellvizio® mucosal assessment
using Cellvizio® ColoFlexTM UHD Miniprobe
Healthy Colon
Visualized with Cellvizio ColoFlex UHD miniprobe
Colon Adenocarcinoma
Visualized with Cellvizio ColoFlex UHD miniprobe
1. Ussui V.M. et al. Confocal Endomicroscopy of Colorectal Polyps. Gastroenterology Research and Practice, 2012.
2. Buchner A.M. et al. Comparison of Probe-Based Confocal Laser Endomicroscopy With Virtual Chromoendoscopy for Classification of Colon Polyps. Gastroenterology, 2010.
3. Shahid M.W. et al. Diagnostic Accuracy of Probe-Based Confocal Laser Endomicroscopy and Narrow Band Imaging for Small Colorectal Polyps: A Feasibility Study. American Journal of Gastroenterology , 2012.
4. Gómez V. et al. Interobserver Agreement and Accuracy among International Experts with probe-based Confocal Laser Endomicroscopy in Predicting Colorectal Neoplasia . Endoscopy, 2010.
5. Buchner A.M. et al. The Learning Curve of in vivo probe-based Confocal Laser Endomicroscopy for Prediction of Colorectal Neoplasia. Gastrointestinal Endoscopy, 2011.
6. Shahid M.W. et al. Diagnostic Accuracy of probe based Confocal Laser Endomicroscopy in Detecting Residual Colorectal Neoplasia after EMR: A prospective Study. Gastrointestinal Endoscopy, 2012.
7. Shahid M.W. et al. Accuracy of Real-time vs. Blinded Offline Diagnosis of Neoplastic Colorectal Polyps using probe-based Confocal Laser Endomicroscopy: a Pilot Study. Endoscopy, 2012.
Cellvizio® I.V.E. with Confocal MiniprobesTM are regulated Medical Device, CE marked (CE 0459) (Class IIa - NB :G-MED) and FDA cleared. Cellvizio® is a registered trademark and Confocal MiniprobeTM is a trademark of Mauna Kea Technologies. Cellvizio® I.V.E. with Confocal MiniprobesTM is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. The use of this medical device is exclusively reserved for healthcare professionals.