Lung Nodules
Cellvizio® is the real-time in vivo cellular imaging platform that enables you to characterize lung nodules with confidence in cases where navigation is difficult or conventional diagnostic screening returns ambiguous results.
Lung cancer is the world’s leading cause of cancer deaths, and its diagnosis remains challenging.1
While lung cancer starts as small nodules in lung parenchyma commonly, early and accurate diagnosis allows timely surgical resection of malignant nodules while avoiding unnecessary surgery in patients with benign nodules.
Cellvizio enables you to classify lung nodules in real-time, in vivo, providing you with the advantage of unique visibility and information.
The National Lung Screening Trial (NLST) showed that screening high risk patients with low dose chest computed tomogram (CT) reduced mortality from lung cancer by 20%. However, 96% of the positive screens were subsequently found not to be malignant suggesting the need for an accurate diagnostic tool with high specificity.2
Challenges and Limitations with Current Standards of Care
Transthoracic needle aspiration (TTNA) is currently the preferred mode for sampling peripheral pulmonary nodules because it has a high diagnostic yield ranging from 80% to 90%.3 However, it has a 12% to 45% risk of pneumothorax with 2% to 15% requiring chest tube placement.4
Flexible bronchoscopy is safer but provides a much lower diagnostic yield (varying from 38.5% to 63.7%) even when using advanced bronchoscope techniques5, as around 80% of the nodules are located at the periphery of the lung.6 This low yield is also a consequence of the lack of positive bronchus sign, as 51.6% of lung nodules do not exhibit a bronchus sign leading directly to the peripheral lung lesion.7 The diagnostic yield drops from 79% with a bronchus sign to 31% without it, creating significant challenges.8
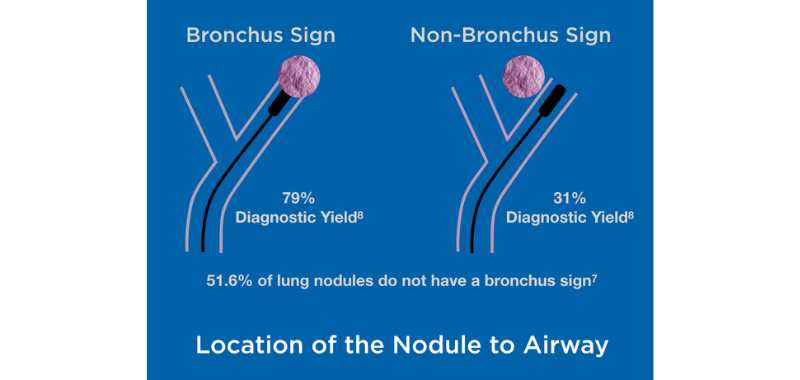
Accurate sampling of lung nodules is challenging not only because of their location to the airway but also because of intratumor heterogeneity, even in early cancer.9 Despite robotic, advanced navigation, fluoroscopy, cone beam CT, and other technologies, these challenges remain.
As today there is a lack of precise periprocedural intralesional assessment mechanism to not only confirm that FNA is sampling from within the lung nodule, but also to ensure that cell or tissue material retrieved with the needle is from the most abnormal area within the lung nodule.
This combination of issues can lead to late or inaccurate diagnosis with consequences on survival rate, as the sooner the cancer is detected the better it can be treated effectively.10
Introducing Cellvizio® needle-based Confocal Laser Endomicroscopy (nCLE)
Cellvizio® is the real-time in vivo cellular imaging platform that can capture images from inside a lung lesion. Deployed through a needle, the nCLE probe enables direct visualization of live cells and their organization from within the lesion, a significant advantage compared to conventional techniques. It will help physicians better target biopsies in the lung.11
Also, by enabling real-time visualization from inside lung nodules and lymph nodes, Cellvizio® nCLE may be used as a feedback technique for diagnostic, staging and treatment procedures in lung cancer.11
In a recently published study, physicians were also able to accurately discriminate nCLE images and videos of lung tumors from airways and normal lung parenchyma (alveoli).12
More importantly, physicians using Cellvizio® nCLE could detect malignancy in tumors and metastatic lymph nodes with an accuracy of up to 95%11,12, leading to better informed patient management.
Live from the clinic at ERS 2020, see the clinical utility of Cellvizio in
characterizing lung nodules in real time like never before.
News about Cellvizio nCLE
Mauna Kea Technologies Announces the Launch of a First in Human Study Combining Robotic Navigational Bronchoscopy and Needle-Based Confocal Laser Endomicroscopy (Clinicaltrials.gov: NCT04441749)
See press release
Lung Lesion Characterization Using Cellvizio® nCLE (AQ-Flex™ 19) Miniprobe
Airways criteria*
- Absence of directional streaming
- Elastin deposits
- Homogenous enlarged cells in an equal distance
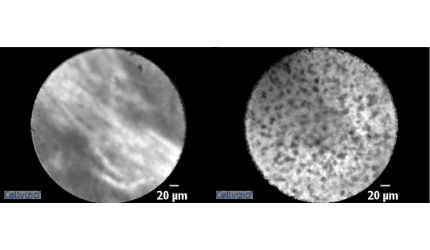
Alveoli*
Alveolar tissue
Necrosis*
Loss of contact signal
For professional investors only: goetzpartners securities Limited issued a report which highlights how interventional pulmonology represents a promising commercial opportunity for Cellvizio®, and is available for download here.
* Images and videos courtesy of Pr. Annema and Dr. Wijmans, Department of Pulmonology, Amsterdam University Medical Center
1. GLOBOCAN 2018, Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. https://doi.org/10.3322/caac.21492 PMID:30207593
2. The NLST Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011 Aug;365(5):395–409.
3. Hiraki T, Mimura H, Gobara H, Shibamoto K, Inoue D, Matsui Y, et al. Incidence of and Risk Factors for Pneumothorax and Chest Tube Placement After CT Fluoroscopy–Guided Percutaneous Lung Biopsy: Retrospective Analysis of the Procedures Conducted Over a 9-Year Period. Am J Roentgenol. 2010 Mar; 194(3):809–14.
4. Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol JVIR. 2010 Jul;21(7):969–75.
5. Ost D.E. et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med, 2017.
6. Heuvelmans, A. Et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer, 2017.
7. Sandeep J. Khandhar, Mark R. Bowling, Javier Flandes, et. al.; Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, mulitcenter NAVIGATE Study
8. Seijo LM, de Torres JP, Lozano MD , et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138 (6) 1316-1321
9. Senosain MF, Massion PP. Intratumor Heterogeneity in Early Lung Adenocarcinoma. Front Oncol. 2020;10:349. Published 2020 Mar 17. doi:10.3389/fonc.2020.00349
10. Cancer Stat Facts: Lung and Bronchus Cancer." National Cancer Institute, https://seer.cancer.gov/ statfacts/html/lungb.html
11. Wijmans L. et al. Needle-based confocal laser endomicroscopy (nCLE) for real-time diagnosing and staging of lung cancer, European Respiratory Journal, 2019.
12. Kramer T, Wijmans L, de Bruin DM, Bonta PI, Annema JT. Bronchoscopic needle based Confocal Laser Endomicroscopy (nCLE) as a real-time detection tool for peripheral lung cancer. Department of Pulmonology, Amsterdam University Medical Center. ERS Presentation 2020.
Cellvizio® I.V.E. with Confocal MiniprobesTM are regulated Medical Device, CE marked (CE 0459) (Class IIa - NB : G-MED) and FDA cleared. Cellvizio® I.V.E. with Confocal MiniprobesTM is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. Please consult labels and instructions for use. Product availability cannot be guaranteed in all countries. For further information, please contact your local sales representative. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. AQ-Flex™ 19 is CE cleared : « AQ-Flex™ 19 Confocal Miniprobes™ are intended to allow imaging of anatomical tracts, i.e., gastrointestinal tracts and respiratory tracts, accessed by an endoscope or endoscopic accessories, including through endoscopic needles. » AQ-Flex™ 19 is FDA cleared: « Once connected to the Cellvizio® 100 Series system, Confocal Miniprobes™, AQ-Flex™ 19, is intended to allow imaging of anatomical tracts, i.e., gastrointestinal and respiratory tracts, accessed by an endoscope, or endoscopic accessories (e.g. aspiration needles used during procedures including EUS-FNA, EBUS-TBNA and TBNA needles) »